ASTM F2582: 测试 Impingement of Hip Prostheses
In total hip arthroplasty (THA), 磨损率降低的累积进展导致颗粒诱导的骨溶解的减少, and wear-related revisions. With this decline, 髋关节不稳定和机械松动已成为翻修手术的主要原因.[1] 然而,脱位及其不可预测性仍然是THA的重大挑战.
推断任何诱发患者的因素是困难的,没有任何显著的统计能力, even with large sample size clinical data, due to the large number of variables. 尽管很少有对照良好的单外科医生长期研究存在, utilizing few changes in implant and surgical technique, the data provide benefits in terms of statistical power but lack the scope of changing key variables of interest. [2]
Hip prostheses challenges
The contributions controlled by the device manufacturer can tremendously influence instability and dislocation. Aside from surgical technique, patient disposition, and patient activity factors, variables can include head size, liner lip chamfer angle, cup insert depth, cup liner offset, or femoral stem offset. This list is illustrative, 不详尽的, to show just how many variables contribute to THA hip instability.
im体育APP通过利用其多样化和广泛的历史来重新关注这些挑战 mechanical testing 具有im体育平台app下载领先的生物医学模拟测试设备经验, as well as a range of custom designed test equipment. With this capability, our orthopedic device experts help customers understand the complex mechanisms of their hip systems for development as well as device clearance through the FDA and other regulatory bodies.
Our approach to impingement testing
Within the past 10-15 years, standardized mechanical test methods for hip impingement were developed to more closely replicate clinical failure modes. While there are many test methods for hip devices, the focus of this article is on ASTM F2582-Standard Test Method for Impingement of Acetabular Prostheses as it pertains to single-piece acetabular prostheses, 模块化假体和由金属制造的约束假体, ceramic or polymeric materials.
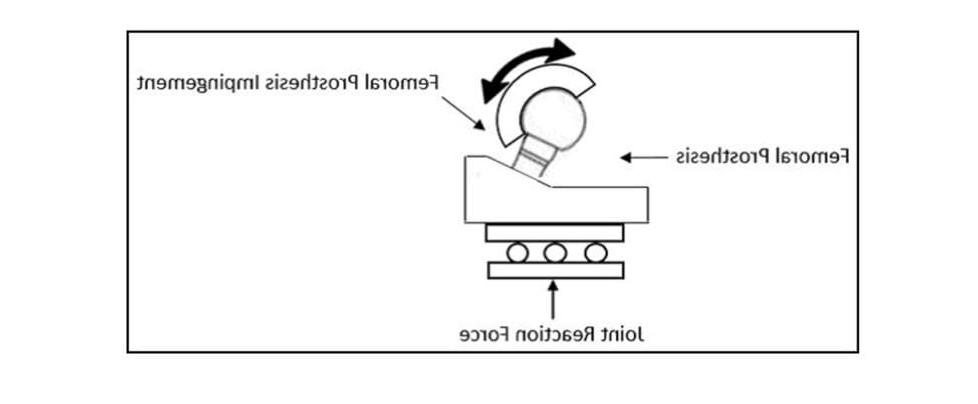
The ASTM F2582 test method involves a displacement controlled test in which the acetabular liner is subject to repeated impingement from the femoral stem, 同时进行生理上相关的关节和负荷. 从阀杆与衬管接触的初始参考位置开始, the system is articulated 0°-5° in abduction, 0°-10° in extension and -5°-5° in internal/external rotation. Figure 1 graphically illustrates the phase alignment of the motions.

除了运动外,还施加了一个恒定的600N关节反作用力. 作用力应与衬垫的入水平面正交, 如图2所示,但随着尾管铰接,也成功地通过阀杆进行了应用, as shown in Figure 3.

Preparing specimens for ASTM F2582 testing
The oxidation of the finished and packaged ultra-high molecular weight polyethylene (UHMWPE) liners and subsequent degradation of chemical and mechanical properties is measured in years. Preconditioning according to ASTM F2003 - Practice for Accelerated Aging of UHMWPE after Gamma Irradiation in Air aids in accounting for any degradation of the liners. The method involves immersing the liners in a pure oxygen environment at elevated pressure and temperature for two weeks.
随后,测量未磨损髋臼衬套的表面几何形状. 测试后进行第二次几何测量,以计算由于撞击引起的体积变化. 这些测量通常是通过结构化蓝光或CT扫描进行的. 创建实体模型,并执行扫描到扫描的比较以确定体积变化.
最后, the liners are preconditioned according to ISO 14242-2, 隔段清洗和称重衬管的标准化过程, while being soaked in a bovine calf serum solution at 37°C. The liners absorb fluid over days to weeks and are repeatedly weighed with this process until a steady rate of fluid sorption is established. 这一步的最终重量测量用作衬垫的测试前质量, and liners are then fitted into test fixtures to begin testing.
Impingement test method
在一般情况下, the testing involves applying a constant joint reaction force of 600N and performing a series of five intervals of 200k cycles each. The femoral stem impinges on the liner rim in abduction motion while also rotating in flexion-extension and internal-external rotation. Between intervals, the impingement position is adjusted or reset to ensure contact between the stem and liner occurs throughout the entire cycle. 该测试通常在37°C的牛血清溶液中进行,频率为1-2Hz, 高达3Hz. 为了进行周期性质量损失分析,在间隔之间去掉了衬里, although this process can be omitted, as discussed further in the challenges and recommendations section.
根据ASTM F2582,总共七个标本通常用于评估髋关节假体. Three of the specimens, termed ‘impingement specimens’, undergo the joint reaction force and abduction-adduction, flexion-extension, and internal-external rotation. Another three specimens are used as articulating controls and are tested in the same condition except they are positioned such that impingement of the femoral stem on the liner never occurs. 最终试样作为加载的对照试样,只承受联合反作用力, allowing the de-coupling of fluid absorption. 类似的, the articulating control specimens allow for the de-coupling of mass loss due to impingement from the mass loss due to wear.
Impingement specimen fixation
In most cases, the acetabular shell is carefully cemented in PMMA bone cement in a Delrin or 316SS fixture. The femoral stems are either full hip stems modified to fit in the test fixtures or machined trunnions specifically designed to fit in the test simulator while still maintaining all of the critical geometry of the final part. In either situation, the femoral stems need to be carefully machined to align the location of impingement with the desired worst-case orientation.
ASTM F2582 challenges and recommendations
There are a few parts of the standard that require knowledge of the purpose for conducting the test and careful stewardship over how the test is carried out. 此外,了解测试能做什么和不能做什么也很重要. 我们将讨论几个需要额外注意和考虑的领域.
其中一个挑战是在间隔检查期间外展角度的调整. During this step, 阀杆以外展运动旋转,直到它撞击到衬管上, 这个位置成为外展运动的新起点. Adjusting the device on the test frame presents challenges due to the small test components and curved surface features, 以及相对较大的外展活动范围,这可能会阻碍对部件的访问. Careful visual inspection and adjustment of the device are crucial. One possibility to make this adjustment more objective would be to rotate the stem in abduction motion until a pre-defined moment is achieved. The rotation could be performed with a 6-axis load cell so that the precise starting point could be located at each interval.
另一个值得关注的领域是在测试过程中相对较小的质量损失. 每百万次循环只需要几毫克. 当试图纠正液体摄取时,质量损失会进一步混淆, 或者把磨损造成的质量损失和撞击造成的质量损失分开. 样本大小只有三个撞击标本和三个关节对照标本, the variation between these two groups is sometimes undetectable.
此外, 如果每20万次循环将衬垫从外壳中取出进行质量损失分析, any small changes that may be present could be obscured. Many times the intermittent removal and mass analysis of the liners is omitted in favor of a simple pre- and post-test measurement. Another reason for omitting liner removal is that a trend of mass loss due to impingement is not as important as the total mass loss upon test completion. 即便如此,磨损造成的质量损失也要按照ISO 14242或类似标准单独进行严格测试.
If interval mass analysis is to be performed, 从贝壳中去除衬里的一种成功技术是利用液氮. 由于聚乙烯衬垫和钛壳的热膨胀率不同, exposing the acetabular components to liquid nitrogen allows for easy removal with no damage to the liner or the locking mechanism. This method works so well that the liner can be removed from the shell with liquid nitrogen without the use of any other tools to pry or force the liner.
最后, ASTM F2582定义的失效标准缺乏可测量的细节来客观地确定衬垫性能. 除此之外,该标准将失效定义为“任何假体部件的总变形”.” The definition is ambiguous and difficult to apply consistently because every system exhibits permanent liner deformation at the site of impingement. 也, in some systems, there is metal-on-metal contact, which can generate a lot of harsh particulates. While this is not addressed in the standard, it is easy to see how damaging this would be in the field. 另外, the failure criteria do not state anything in regards to the mass loss analysis or volume difference measurements and how to interpret these results. These measurements are simple to report, 但是对这些结果的意义的解释还需要进一步的分析.
结论
As impingement of the stem and liner is imminent in-vivo, 表征和理解髋关节假体在这种情况下的表现是很重要的. ASTM F2582方法并不旨在准确地重建体内条件. 它, 然而, provide a standardized means for assessing the functional performance of an acetabular prosthesis under laboratory conditions. Even without clear failure definitions, 该测试方法有助于系统地研究不同的假肢设计考虑. Depending on various goals, investigators may choose to deviate from the test method to look at other failure modes or test parameters in more detail.
参考:
[1] 凯文·博齐克.等。. 美国翻修全髋关节置换术的流行病学.[JBJS.1 (2009): 128-133.
[2] Brown, Thomas D et al. 全髋关节置换术中的撞击和脱位:机制和后果.” The Iowa orthopedic journal vol. 34 (2014): 1-15.
髋臼假体撞击的标准试验方法
The im体育APP advantage
Our 从事专家 excel at orthopedic device testing and have worked with many challenging device designs and test setups over the last several decades. 我们努力以最方便的方式满足您的所有医疗器械测试需求, efficient and responsive way. im体育APP to discuss how we can help with your test project.
找到相关的 资源
Get white papers, updates and event invites
Subscribe to content updates
Related Services

Orthopedic Implant 测试
As a global leader in orthopedic implant testing, im体育APP has years of experience in evaluating hip replacements, knee prostheses, spinal devices and many other implants.

Preventing Fretting Corrosion of Orthopedic Devices
人体是一个腐蚀性的环境,可以损害矫形装置的性能. 本文概述的测试方法有助于防止设备设计中的微动腐蚀.

Test Protocol for Medical Devices
A protocol and plan will mitigate your risk, prevent confusion, set clear expectations, and preserve the necessary information for future reference and use.

Biomechanical 测试 of Cadaveric Specimen
Cadaveric biomechanical testing is one of the most beneficial forms of mechanical testing in the medical industry. 我们的医疗设备测试专家解释了尸体标本测试的优势和挑战.
